
In the previous two reports, we discussed how different farming systems and how we use synthetic nitrogen fertilizer can have a major effect in controlling greenhouse gas (GHG) emissions as part of a Sustainable and Sustainable Intensive Agricultural System (SA/SIA).
We’ll now explore in more detail some of the products and techniques available to assist.
Greenhouse Gas Emissions Are Only Part of the Story
Although, so far, we have concentrated on GHG emissions (particularly carbon dioxide and nitrous oxide), from both manufacture and use, it’s important to recognise that the incorrect use of synthetic nitrogen fertilizer can have other damaging effects, which are also relevant to SA and SIA. Nitrogen that is not taken up by the plant can end up causing harmful pollution. This occurs by leaching, volatilization and, sometimes, by runoff.
Leaching is the process whereby, through gravity, nitrogen is unavailable to the plant roots and is carried to the groundwater supply primarily as nitrate (NO3-). This naturally occurs from soil organic matter but can hugely increase from nitrogen fertilizer applications (both synthetic and manure). This is generally a more serious problem on lighter soils with a higher sand content, or where the water table is at shallow depth.
For nitrogen leaching, the principal problem is anoxia (or internal suffocation), a condition that affects pregnancy and early childhood in both humans and animals; this is sometimes called “blue-baby” syndrome. For nitrogen volatilization, this is the process whereby the applied fertilizer is lost to the atmosphere, primarily as ammonia (NH3) gas. This process is more pronounced on alkaline-rich soils (high pH) and at higher temperatures.
Agriculture is a major contributor to the polluting NH3 emissions. The level has doubled in the last 60 years. There are multiple damaging effects once this has been converted to ammonium (NH4), with their fine particulate matter (PM 2.5 microns or less) associated with smog, cancer, heart disease and much more. NH3 is not categorized as a GHG…
There is clearly a lot to think about when considering a workable SA and SIA program.
The Four “R’s”
There are a couple of concepts which are useful in considering nitrogen fertilizer use.
The “Four Rs” are a frequently quoted set of practices, which influence emissions and are usually applied to N2O emissions by everyone, from manufacturers to environmentalists.

Right Formulation
Farmers have a wide range of choices when it comes to fertilizer, depending on their crops and global regions. The most widely used nitrogenous fertilizer is urea, accounting for more than 50% of global production. In the US, for example, Anhydrous Ammonia is popular and, on cereals, has a 30-40% market share. Europeans and Russians are fond of Ammonium Nitrate. Chinese producers favour urea, and so on.
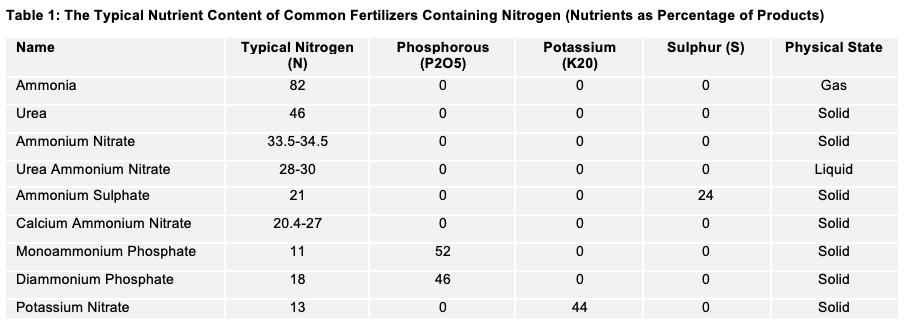
Choosing the right formulation is a balance between availability, intended use, cost and hopefully more in the future on environmental profile. For example, urea works well in cool conditions, as it releases its nitrogen depending on soil temperature (i.e., when the plant is growing in warmer soil). It also has high levels of undesirable volatilization in warm and hot climatic conditions, where ammonium nitrate may be a more suitable choice. Ammonia (aqueous) is inexpensive but, in some circumstances, can be environmentally more harmful than other choices.
The University of Michigan (Bulletin E3152) reports that, in corn (maize)-soybean rotations, N2O emissions can be two to four times greater following Anhydrous Ammonia than Urea and Ammonium Nitrate (UAN) or Broadcast Urea. It also stated that “the trend towards more urea in corn in the United States may help reduce N20 emissions”. Even with Anhydrous Ammonia, there are some field choices that we’ll consider under Right Placement later, such as the effect of soil depth placement on the application.

Best choices will also be influenced by the type of nitrogen and performance in different real-life conditions. For example, Urea and Ammonium Nitrate is typically 75% NH4+ (Ammonium) and 25% NO3- (Nitrate).
Although rarely discussed, judicious use of nitrogenous fertilizer with other nutrients (N, P, K and S) can also be helpful on some soil types and systems as they can reduce the number of field passes and thus reduce diesel or other fuel usage.
Nitrification Inhibitors and Stabilizers
We already demonstrated in the previous report that, in the model we were using, nitrification inhibitors can reduce N20 emissions by 32.5%, although figures up to 50% are possible.
Urease (UI) and Nitrification Inhibitors (UI/NI) work in different ways. UI blocks urease enzymes, which delays the process of converting Urea into Ammonia. NI, the important one for GHG emissions, delays the conversion of Ammonium into Nitrate, therefore reducing leaching and critically substantially reducing NO2 emissions. Another possibility is slow-release fertilizers by applying a sulphur, resin or polymer coating with the purpose of providing nutrient to the plant as needed. The case is still out on how significantly these slow-release benefits will improve GHG emissions, but theoretically, they should do.
Farmers and scientists are still debating the benefits of UI/NI as a lot of the technology is fairly new. Superficially, these types of products are too expensive for row crops, although in horticulture, they have gained traction. There is some (limited) evidence that yields can increase by the more efficient use of the nitrogen, and by enough to more than offset the cost. They can be used on all the nitrogen fertilizers (seen in Table 1), including Aqueous Ammonia.
Encouragement or Enforcement?
Legislators also influence choice. This can vary from. For example, the very blunt instrument of the UK Government, which proposes to ban Urea because of its delayed late season use when early spring conditions are wet (see above why this is a problem because of volatilization). The net effect could easily be to reduce NH3 emissions at the expense of deteriorating water quality from other sources of Nitrogen, such as Ammonium Nitrate. Why not place a restriction on date-of-use, encourage swaps, unused return schemes (it can be stored) after the cut-off, or exempt nitrification inhibited urea?
Schemes in other countries, such as Canada and Switzerland incentivize this. They provide farmers with tradable carbon offset credits for good practice.
Conclusion
Choosing the right fertilizer from the factory is the first of the Four Rs, and it’s an important one. Next time, we’ll see what happens on the farm with the remaining three Rs.

Other Opinions You Might Be Interested In…
- NEW! Sustainable Agriculture: Origins and Evolutions
- Sustainable Intensive Agriculture: A New Approach
- Sustainable Agriculture: Arable Farming
- Sustainable Agriculture: Arable Nitrogen Use
- Which Fertiliser is Cheapest Today? 25th November 2020

